How to Read a Pet Scan Cd
- Review
- Open Access
- Published:
How nosotros read pediatric PET/CT: indications and strategies for epitome acquisition, interpretation and reporting
Cancer Imaging volume 17, Article number:28 (2017) Cite this article
Abstruse
PET/CT plays an important function in the diagnosis, staging and management of many pediatric malignancies. The techniques for performing PET/CT examinations in children have evolved, with increasing attending focused on reducing patient exposure to ionizing radiations dose whenever possible and minimizing scan duration and sedation times, with a goal toward optimizing the overall patient experience.
This review outlines our arroyo to performing PET/CT, including a discussion of the indications for a PET/CT exam, approaches for optimizing the exam protocol, and a review of different approaches for acquiring the CT portion of the PET/CT exam. Strategies for PACS integration, image display, interpretation and reporting are besides provided.
Most practices will develop a strategy for performing PET/CT that best meets their corresponding needs. The purpose of this article is to provide a comprehensive overview for radiologists who are new to pediatric PET/CT, and too to provide experienced PET/CT practitioners with an update on state-of-the art CT techniques that we have incorporated into our protocols and that take enabled us to make considerable improvements to our PET/CT practise.
Introduction
Positron Emission Tomography/Computed Tomography (PET/CT) plays an of import role in the diagnosis, staging and management of a broad range of pediatric malignancies including Hodgkin and non-Hodgkin lymphoma, malignant soft tissue and bone sarcomas, head and cervix tumors, Langerhans prison cell histiocytosis (LCH) and neuroblastoma [1,two,3,four]. PET/CT is the almost common hybrid imaging technique currently in use, owing to the increased sensitivity and specificity of PET/CT for detecting metabolically agile malignancies. 18Fluorine-2-fluoro-2-deoxy-d-glucose (FDG) is still the well-nigh commonly used radiopharmaceutical in routine clinical use, only a number of new PET tracers are being developed with potential for use in imaging children with cancer.
In addition to the discovery and development of novel radiopharmaceuticals for detection of malignant disease, the techniques used to acquire PET images have also evolved. PET imaging was initially confined to review of emission imaging data. Non-diagnostic quality manual scans were obtained only for soft tissue attenuation correction of the PET raw data, but provided no additional anatomic information. With the development of integrated hybrid PET/CT scanners, many new opportunities emerged for obtaining high quality co-registered CT images [5]. Considering we at present have numerous options for obtaining CT images as office of the PET/CT acquisition there is an increasing need for sensation and selection of appropriate CT imaging techniques. These CT techniques may vary substantially, and are largely dependent on whether the CT images are intended for attenuation correction only, anatomic co-localization, or diagnostic interpretation [6].
This review will outline our approach to performing PET/CT in children with a variety of pediatric cancers. Nosotros volition review current indications and common practices for using PET/CT and the evidence supporting these practices, and talk over the applied aspects of performing and interpreting PET/CT examinations in children.
Indications
Indications for performing (and reimbursing) PET/CT differ between countries and healthcare systems. In the US, reimbursement programs also differ between states and betwixt insurance companies. While private insurers may exercise more breadth in considering payment for imaging pediatric cancers, well-nigh third political party payers initially rely on the national coverage determinations provided by the Centers for Medicare and Medicaid Services (CMS) for guidance. CMS has expanded the reimbursable indications for employ of PET/CT to include the majority of adult cancers [vii]. In some cases there is overlap with pediatric cancers, such as lymphoma and melanoma, although for the majority of pediatric malignancies CMS does not provide explicit guidance or approval. Equally such, in most pediatric cancer centers such equally ours, insurance pre-authorization is sought for most all new cancer diagnoses prior to obtaining a PET/CT, whether for initial staging or for interim response assessment. CMS approved indications, for which specific reimbursement codes be, include both Hodgkin and non-Hodgkin lymphoma, melanoma, neuroendocrine tumors, and Langerhans Cell Histiocytosis, besides every bit other malignancies more commonly seen in adults but occasionally occurring in children (e.g. colorectal and esophageal cancer). In the instance of Hodgkin lymphoma, the use of FDG PET/CT to evaluate early metabolic response to therapy has led to new response-based treatment algorithms and completely changed the approach therapy (Fig. ane) [iii, 8].
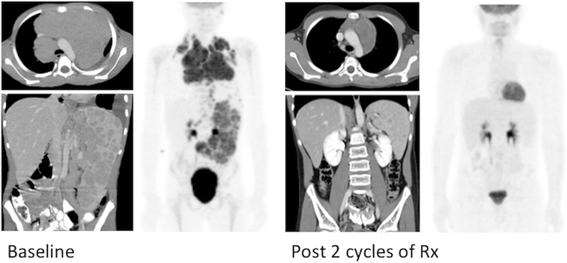
PET/CT in Hodgkin Lymphoma. Baseline PET/CT shows beefy mediastinal, extensive splenic and intra-intestinal nodal disease. Following 2 cycles of chemotherapy the 18F-FDG PET/CT shows a complete metabolic response to therapy, whereas remainder mediastinal soft tissue mass and splenic lesions remain. Following a response-based treatment algorithm, subsequent therapeutic decisions are fabricated based on the metabolic consummate response (CR) shown past PET/CT
In many other pediatric cancers, including Ewing sarcoma, rhabdomyosarcoma, synovial cell sarcoma, osteosarcoma, gastrointestinal stromal tumor (GIST) and MPNST, in that location is accumulating data showing the importance of PET/CT in staging, and in some cases response assessment. For the staging of osteosarcoma, Ewing sarcoma and rhabdomyosaroma there is consistent testify of improved sensitivity of PET/CT for detecting skeletal metastases when compared to conventional techniques such equally bone scintigraphy [2]. The data is less clear regarding the utility of PET/CT for assessing response and predicting outcome in the cancerous sarcomas, although several small studies take shown a correlation betwixt changes in FDG uptake (standardized uptake value, SUV) and response to therapy and outcome [9,10,11]. Pediatric malignancies are comparatively rare, making it difficult to design big clinical trials to demonstrate the utility of FDG PET/CT in the management of most pediatric cancers. As of nevertheless no prospective trials take incorporated response-based handling decisions into algorithms that rely solely on changes in FDG uptake to dictate course of therapy, which limits our assessment of the prognostic value of PET in these malignancies.
In neurofibromatosis type-1, FDG uptake is an effective biomarker for predicting evolution of benign neurofibromas into either premalignant atypical neurofibromas or cancerous peripheral nerve sheath tumors (MPNST) [12]. Many other pediatric cancers have been shown to be FDG-avid and a staging PET/CT has been shown in many small studies to have improved sensitivity over existing techniques [iv, 13, 14]. In contrast, neuroblastoma, despite being the virtually common non-CNS solid tumor occurring in children, is non routinely imaged by FDG PET/CT, owing generally to the large torso of evidence showing the value of 123I-MIBG for staging and predicting outcome subsequently response to induction therapy, in addition to establishing the extent of disease prior to beginning handling with 131I-MIBG [fifteen, 16]. In add-on, in many cases neuroblastoma is FDG-negative and equally such the routine use of FDG PET in staging and response assessment in neuroblastoma has been largely restricted to those few patients with MIBG-negative illness [17]. Wilms tumor is the well-nigh common renal tumor occurring in babyhood, although physiologic excretion of FDG from the kidneys has limited the routine use of PET/CT for management of Wilms tumor [eighteen]. In our feel, notwithstanding, PET imaging may withal be useful for staging, peculiarly for characterizing extrarenal sites of affliction, and for re-staging at the time of relapse.
Unique molecular targets are too being identified for many pediatric malignancies. Tumors such as inflammatory myofibroblastic tumor (IMFT), as well as other uncommon pediatric neoplasms, are receiving renewed attention based on the identification of molecular markers against which targeted therapies can exist directed. The utilize of FDG PET/CT to monitor changes in tumor metabolic action in response to treatment with molecularly targeted agents, may be an of import surrogate for establishing pharmacologic activity of new drugs being evaluated in early phase clinical trials [19]. In detail, alterations in tumor metabolic activity tin play an important role in guiding therapy, even in when significant measureable changes in lesion size and/or number are not observed (Fig. 2).
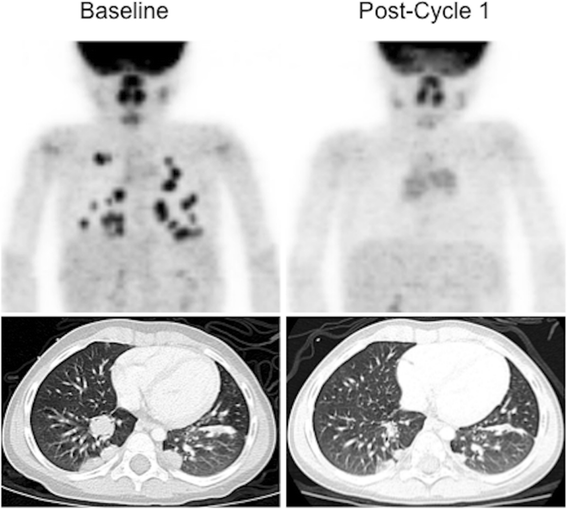
PET response to targeted experimental Phase 1 therapy: Crizotinib in ALK+ IMFT. 18F-FDG-PET and CT imaging of Crizotinib response in IMFT: Baseline whole trunk xviiiF-FDG-PET and Chest CT testify multiple FDG-avid pulmonary nodules, confirmed by biopsy to be IMFT. Post-obit one bicycle of therapy, no rest abnormal FDG aggregating is seen (metabolic CR). The lesions have decreased significantly in size past CT, coming together criteria for fractional reponse (PR), merely not CR. This patient has remained gratuitous of disease for >36 months [32].
Practical aspects of PET/CT: ordering, protocoling, acquiring, and interpreting the PET/CT examination
Ordering a PET/CT
PET/CT is typically performed as either an exam of the whole torso or the torso (optics-to-thighs), although more focused limited examinations may be specified by the ordering physician. In general, lymphoma patients undergo examinations of the torso, which assures coverage of the majority of the lymphoid tissue, extending from Waldeyer's ring through the inguinal lymph nodes. Because of the potential for metastatic disease occurring anywhere in the body, sarcoma patients unremarkably undergo a whole-trunk PET/CT. For other patients the extent of coverage should be determined in conjunction with the ordering clinician to ensure adequate coverage of specific regions of involvement based on the patient's disease, and to be in compliance with coverage adamant at the fourth dimension of insurance pre-authority. Some 3rd party payers will approve a PET/CT of the trunk, but deny coverage for a whole-body exam.
At the time a PET/CT study is ordered, the referring clinician should determine if a diagnostic quality CT (Dx CT) is required or whether a low-dose CT for anatomical correlation will suffice [20]. To assist clinicians in ordering the right examination, an algorithmic approach is useful (Fig. 3). If a diagnostic study is required, the necessary extent of torso coverage must be conspicuously stated by the referring md. Test techniques and parameters must then be clearly delineated by the protocolling radiologist. For instance, if a diagnostic abdomen and pelvis examination is required, the Dx CT should exist protocoled with intravenous and oral contrast media, using established departmental guidelines. The same is true for a Dx CT of the entire body (Cervix/Chest/Belly and Pelvis). If only a diagnostic chest CT is required, a decision must exist made whether the CT chest is for the purpose of characterizing mediastinal adenopathy and soft tissue disease, in which case Four dissimilarity media is required, or for the identification and characterization of pulmonary nodules. In this latter case, a non-contrast protocol is typically used and the test is performed at end-inspiration to optimize visualization of small lung nodules.
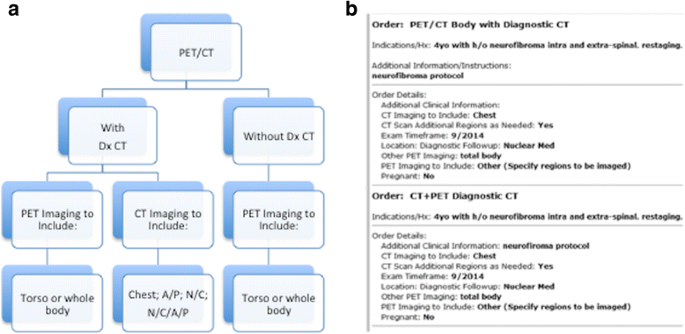
Guide to ordering a PET/CT together with diagnostic CT imaging. An algorithmic approach (a) to ordering a PET/CT exam provides clinicians with prompts for specific information that guides the ordering procedure and encourages an integrated approach to protocoling both the PET/CT and the diagnostic CT exams. The subsequent order (b) contains the necessary clinical information to allow both the PET/CT and the diagnostic CT examinations to be correctly protocoled
If a Dx CT is not required, the CT portion of the PET/CT examination is protocoled using the lower dose attenuation correction CT (Air conditioning CT, lowest dose) protocol. Many departments distinguish between an AC CT and an anatomic co-localization CT (slightly higher CT dose, but not every bit high as a diagnostic CT), and a diagnostic quality CT. We simply have a unmarried AC CT protocol, with slighter higher tube current for patients > 55 kg, as compared to smaller patients < 55 kg (see subsequently), and routinely utilize Four contrast for the Ac CT, to improve anatomic localization and epitome quality despite the lower CT dose. Importantly, referring clinicians should understand that the low dose Ac CT, while not considered to be of diagnostic quality, nonetheless contains images that in many instances are comparable to diagnostic scans (Fig. 4). Every bit such, it is our practice to routinely issue a separate report summarizing any pertinent findings detected on the Ac CT. This assures a rigorous review of all the patient's imaging data, and in some instances, may identify an unexpected finding that alters patient management (Fig. v), which is consistent with reported rates of 3-5% for clinically significant incidental findings identified on AC CT images [21].
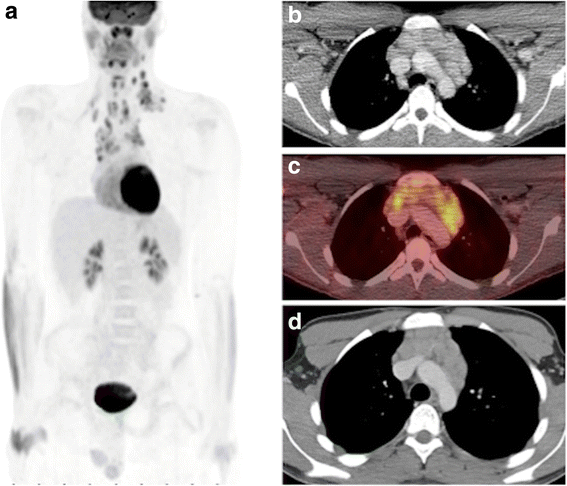
Contrast enhanced low dose attenuation correction CT from PET/CT exam compared to diagnostic CT. The depression dose attenuation correction CT, performed with 4 dissimilarity (b, c) provides anatomic localization of the PET findings (a), and – while not of diagnostic quality – is comparable to the diagnostic CT (d), and has sufficient diagnostic information to warrant a thorough review
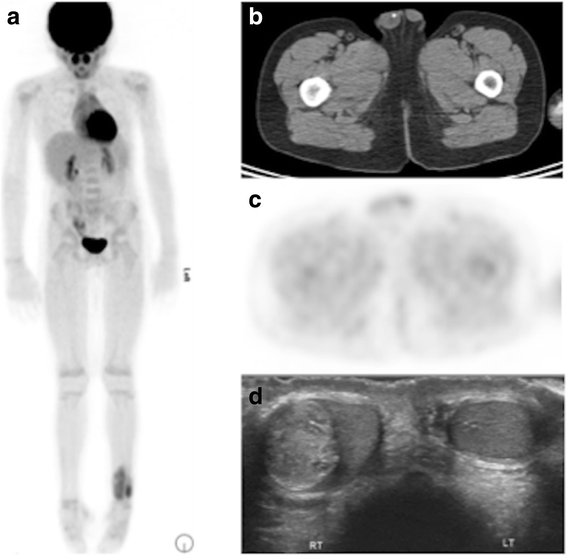
The attenuation correct CT has diagnostic value. Eight years old patient with Ewing sarcoma of the left distal tibia. xviiiF-FDG PET/CT shows uptake in the primary tumor, but no metastatic disease (a). Scrotal calcification was detected incidentally on the AC CT (b), just was not associated with FDG uptake (c). Ultrasound confirmed a mass (d), which was revealed to exist a mature teratoma, unrelated to the chief tumor
Patient preparation
Patients being sedated for the PET/CT take nothing by mouth (NPO) after midnight the night before the written report. For studies being performed without sedation, patients should be NPO at least 4 h before the examination. In all cases the patients remain fasting during the 1-h FDG uptake period. Because insulin secretion is stimulated by caloric intake, high levels of FDG uptake tin occur in both skeletal and cardiac musculus, limiting the interpretability of the PET browse. NG tube feeds, parenteral nutrition, and all dextrose-containing Iv solutions should also be discontinued. If Four hydration is required a elementary isotonic solution of normal saline is preferred. Note that even non-caloric bogus sweeteners such as Nutrasweet® can stimulate insulin secretion and are contraindicated prior to performing FDG PET/CT [22]. Strenuous practice is also avoided for 24 h prior to the exam to minimize background uptake in skeletal muscle.
The possibility of pregnancy must be considered in all post-pubertal females. Fifty-fifty though the likelihood of pregnancy is very small-scale in oncology patients due to ongoing chemotherapy and the underlying oncologic illness, well-nigh institutions accept a policy that requires anyone undergoing a CT examination (and past extension a PET/CT test) to take a negative pregnancy test prior to the procedure. In our establishment, all post-menarcheal female patients age 12 and older undergo urine pregnancy testing prior to the PET/CT exam only if they are also having a diagnostic CT of the abdomen or pelvis; pregnancy tests are non obtained for routine PET/CT studies. The practical and ethical considerations that nourish this policy, particularly regarding patient confidentiality for older teenage patients and procedures for responding to a positive pregnancy test, are beyond the scope of this review and must be individualized for each institution.
Diabetic patients crave special instructions when preparing for an FDG PET/CT examination [22]. Having a skilled nursing team available to communicate with the patient and family several days prior to arrival will help to avert either a cancelled examination or an un-interpretable study. Diabetic patients (whether type one or 2) tin have high circulating glucose levels, either due to absence of insulin production (type 1) or insulin resistance (type 2). Ideally diabetic patients should be scheduled early in the forenoon. Type 1 patients crave exogenous insulin to maintain basal levels of insulin, even while fasting. The long acting form of insulin (NPH), when given around bedtime the prior evening, should be sufficient to maintain advisable insulin levels until the examination is complete. Blood glucose levels must be checked prior to tracer injection. If higher than 200 mg/dl the examination should probably non be performed as high serum glucose levels can compete with the trace amounts of FDG existence administered, thereby reducing the sensitivity of the PET/CT examination.
Brusque acting insulin should not be administered prior to, or during the PET examination as insulin-mediated musculus uptake volition occur, and may limit interpretability of the browse. Many patients with type ane diabetes mellitus employ an insulin pump, and any amending in the insulin-pump regimen should exist fabricated in consultation with the clinician who routinely helps the patient and family manage the insulin pump. Although type 2 diabetes is less mutual in children, its prevalence is increasing in pediatric populations. Patients with type ii diabetes mellitus may exist treated with metformin, which is associated with undesirable colonic, hepatic and muscle uptake of FDG. Ideally, metformin is discontinued for at to the lowest degree two-3 days prior to the FDG PET examination, although this may not be possible if information technology will effect in unacceptable hyperglycemia. In rare instances it may exist necessary to suppress myocardial FDG uptake (due east.g. cardiac and pericardial tumors). This can be achieved by restricting the patient to a loftier-fat ketogenic diet, although in practise we employ this technique primarily for non-oncologic cardiac imaging applications.
For patients who are having a Dx CT equally office of their PET/CT examination, special care must exist taken when administering Iv and oral contrast agents, to ensure the high diagnostic quality of the CT examination. To the interpreting radiologist, a Dx CT that is obtained on the PET/CT scanner as part of the PET examination should be equivalent in quality to a CT performed on a comparably equipped standalone CT scanner. For oral contrast media, nosotros routinely apply between 90 and 300 ml (adjusted by patient age) of contrast, prepared as a one:30 solution of not-ionic iodinated contrast (Optiray 320, Ioversol, Liebel-Flarsheim Company LLC, Raleigh, NC) diluted into water: for example a 10 yo child will receive 180 ml of contrast, prepared as 6 ml of Optiray 320 diluted into 180 ml water. Although many children may adopt contrast diluted in juice, most juices contain sweeteners and should be avoided. In do, the non-ionic contrast, when diluted into water, is tasteless and generally well-tolerated. Most dilute barium preparations (BaroCAT) and other palatable oral contrast media incorporate sweeteners and should be avoided.
Sedation
For patients requiring sedation, boosted direct communication with the sedation or anesthesia squad should take place prior to performing the PET/CT test. This will ensure that any fasting or feeding requirements, and the administration of enteric contrast, is in accord with anesthesia guidelines. We generally wait approximately threescore min after the last administration of enteric dissimilarity before sedating a patient, thereby reducing the chance of an aspiration event that can occur in a patient with a full stomach. The sedation squad as well should be aware of the need for all IV fluids, including Iv medications in saline solutions, to be glucose/dextrose-gratis.
Sedated patients typically receive continuous Iv fluids both prior to and during the exam. As a result, the bladder may go quite full and in small children, as well every bit in children with pelvic neoplasms, excreted FDG in a very full bladder tin obscure areas of involvement and concern, and thereby compromise the examination. Placement of a bladder catheter is ideally done after consultation with the referring oncology squad, although in practice catheter placement tin be accomplished apace and without incident. In neutropenic patients, who are at increased risk for development of infection, and in patients with hemorrhagic cystitis, placement of a bladder catheter may be contraindicated, and the conclusion to place a catheter should exist fabricated only after conferring with the ordering oncologist.
PET/CT acquisition
Although this review is focused on PET/CT, it should exist noted that PET/MR has been shown to result in substantial reductions in radiation dose to the patient, primarily due to the elimination of the CT component of the PET/CT [23, 24]. Increased sensitivity of newer generation solid state PET detectors and longer PET acquisition times during the PET/MR test can farther contribute to dose reduction by allowing for lower administered activities of PET radiopharmaceutical. In some instances PET/MR may be preferable, especially when anatomic co-localization of illness evident on MRI cannot be accomplished past CT [25]. In almost all cases, assuming a portion of the MRI is being performed for diagnostic purposes and not but for attenuation correction, the combined PET/MR examination will require considerably more than time (upwards to iii times longer, depending on the protocol) than a PET/CT providing comparable coverage.
We take recently published a survey of 19 North American institutions where a big volume of pediatric PET/CT examinations are performed [5]. There was surprising variability in do betwixt institutions, particularly when the PET/CT was ordered together with a diagnostic CT. What follows is a description of the approach adult at Boston Children's Hospital for operation of PET/CT, with an aim toward optimizing the scanning technique to avert duplicate CT scanning over the same coverage area and to provide improved anatomic particular when needed to correlate with findings on the accompanying FDG PET scan.
Attenuation correction CT
The attenuation correction CT portion of the PET/CT examination is usually obtained prior to the PET conquering. Attenuation correction accounts for differences in the location of positron annihilation events and the degree to which tissues "attenuate" the PET anything photons (Fig. 6). Photons emitted from the center of the patient must pass through more attenuating tissue, and thus are less likely to be detected than those from the periphery. Furthermore, photons emitted from tissues with minimal attenuation (i.e. air/lung) are more likely to reach the detector than photons emitted from locations within or about dense structures similar os. A more extensive give-and-take of the physics underlying the utilize of CT for PET attenuation correction is beyond the scope of this review and can be found in the recent review past Fahey et al., and references therein [v].
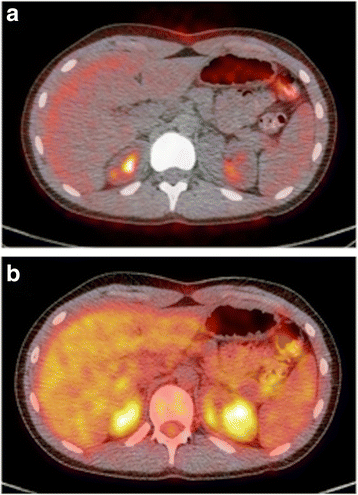
CT based attenuation correction of PET images. Low dose attenuation correction CT exams are non more often than not used for routine diagnostic interpretation. The AC CT accounts for differences in location/depth from which the 511 keV PET photons are emitted, and can exist used to correct for differences in surrounding tissue density (HU density) and the degree to which those tissues blot or attenuate the PET photons [5]. a shows the fused, uncorrected PET data, with increased point at the periphery of the image and poor signal from eye of the body and side by side to the vertebral column. The attenuation corrected image of the aforementioned PET information (b) provides a more accurate representation of the xviiiF-FDG uptake
Traditionally, the CT component of a body PET/CT examination had been performed at our institution as a low-dose non-diagnostic scan using i of two weight-based low dose CT protocols. When a Dx CT was likewise requested, this ofttimes resulted in indistinguishable imaging of the same coverage area, with every bit much as 25-l% boosted CT dose (Fig. vii). By integrating the non-Dx and the Dx CT data there was an opportunity to eliminate duplicate scanning over regions where both CT's were being acquired. The Dx CT provides diagnostic quality information for anatomic correlation and can also be used for attenuation correction [20]. Nonetheless, two concerns were frequently expressed: 1) Iv contrast media used for diagnostic CT changes tissue attenuation and thereby might touch on the attenuation corrected PET information and SUV calculations, and ii) existing software merely allowed for a unmarried series CT conquering during the PET/CT. If merely a Dx Abdomen/Pelvis CT was required, there was no means of merging the Dx CT and low dose Air conditioning CT data in a unmarried reconstructed data set.
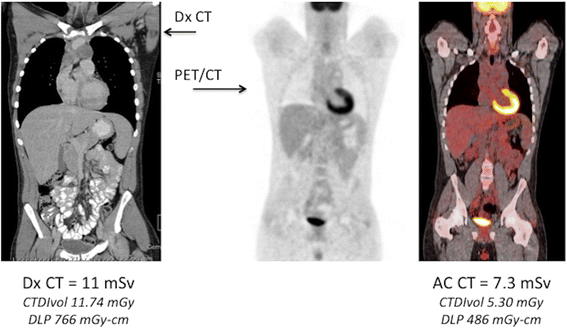
The Double Torso Exam: PET/CT + Diagnostic CT. Dx CT and PET/CT exams performed on the same patient with Hodgkin lymphoma. Both examinations were performed during the same appointment, post-obit 2 cycles of chemotherapy, and show similar coverage between the two CT exams. In this example, the Air-conditioning CT dose is approximately 2/3 of the Dx CT dose. Eliminating one of the two CT exams and performing a single CT, offers an opportunity for significant CT dose reduction
To address these concerns, others have shown [26, 27], and we have verified (Fig. eight) that Iv dissimilarity does not significantly bear upon either SUV max or SUV mean calculations. We then worked with our PET/CT manufacturer (Siemens Healthineers, Hoffman Estates, IL, USA) to develop and validate the conquering software to allow for obtaining a multi-series CT acquisition every bit part of the PET/CT exam, such that the patient receives merely the dose required for each surface area, with minimal overlap. For example, every bit shown in Fig. nine, a non-diagnostic attenuation correction scan can be caused from the skull base of operations through the thorax, followed by a diagnostic scan of the abdomen and pelvis, finishing with a non-diagnostic low dose browse to the mid-thighs to friction match the remaining PET bed position. These separate series are so merged into a single data gear up, which can be used for attenuation correction, anatomical correlation and diagnostic interpretation. This multi-serial PET/CT approach is at present routine for the bulk of our PET/CT acquisitions that require both diagnostic and non-diagnostic CT exams.
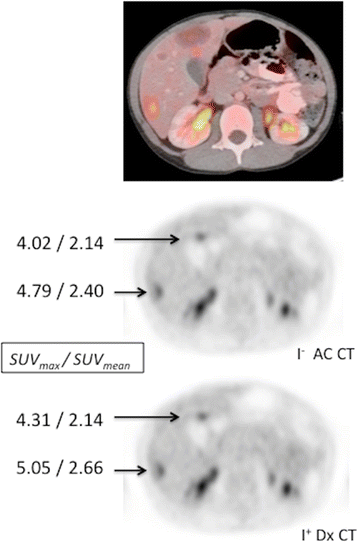
Contrast enhanced Dx CT for PET attenuation has negligible touch on SUV. Not-contrast low dose AC CT and contrast enhanced Dx CT were used to perform attenuation correction on eighteenF-FDG PET images from a half dozen yo child being treated for Burkitt lymphoma. A representative PET image of the liver, fused to the accompanying Dx CT, shows multiple FDG-gorging liver lesions. PET images obtained following attenuation correction using the non-contrast Air conditioning CT and the contrast enhanced Dx CT show negligible differences in SUV values (SUVmax/SUVmean) calculated for representative liver lesions
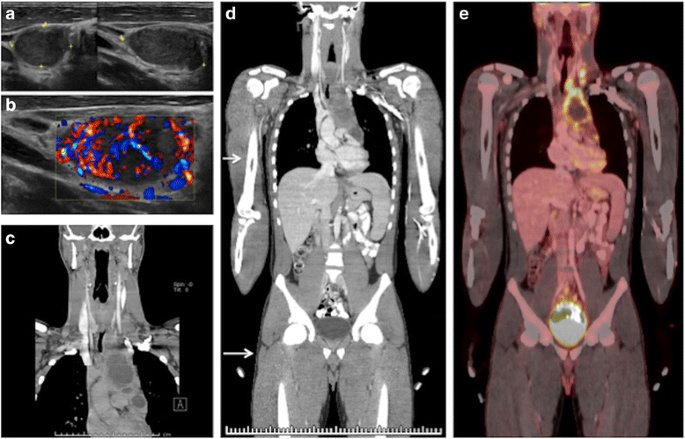
Multi-series PET/CT with Dx Abdomen/Pelvis CT. Xviii years old patient with cervical adenopathy was evaluated initially by U.s. (a, b). Subsequent evaluation included a diagnostic CT of the neck and chest (c). Biopsy revealed Hodgkin lymphoma. Completion staging involved a diagnostic CT of the abdomen and pelvis, which was incorporated into the PET/CT acquisition (d, e), to avert echo diagnostic CT imaging of the neck/breast and double scanning of the belly and pelvis. Arrows (d) show the junction between low dose Air-conditioning CT and diagnostic portions of the exam
Tables i and two provide details for the attenuation correction and diagnostic CT parameters used during PET/CT exams.
As described before in the "Ordering the Examination" section, and shown diagrammatically in Fig. 3, the post-obit imaging algorithm is now routinely used:
- 1)
Decide whether the PET/CT examination is to exist done with or without a diagnostic CT
- 2)
Determine what the PET coverage volition exist (Torso, whole body, or limited).
- 3)
For PET/CT without a Dx CT, follow the arroyo outlined in Table i, with weight based tube current modulation. We routinely apply Four dissimilarity for the attenuation correction CT, with a dose of 2 cc/kg and a charge per unit of 2 cc/sec. Centric, sagittal and coronal reconstructions are generated, in addition to axial reconstructions using a lung algorithm.
- four)
For a PET/CT with a Dx CT, the ordering clinician must specify the CT coverage. Regardless of the Dx CT coverage, the patient preparation (4, oral contrast), weight-based reference mAs, pitch and reconstruction techniques should match the standard Dx CT protocols in apply on other CT scanners in the department. These are summarized in Tabular array 2.
Based on the Dx CT coverage, 1 of three acquisition techniques are used:
- a)
PET/CT with a divide Dx CT (for example, an non-contrast Chest CT to evaluate for lung nodules is performed separately from – usually before – the PET/CT examination given the need for end-inspiration CT imaging and no need for 4 contrast for the diagnostic CT exam).
- b)
PET/CT with a Dx CT of the same coverage (Trunk CT is used both for diagnostic interpretation and PET attenuation correction). This scenario is mutual for lymphoma patients.
- c)
PET/CT with a Dx CT limited to a specific region of involvement that tin can be readily incorporated into the PET exam. This scenario is common for lymphoma patients who may take had a Dx neck and chest CT at the fourth dimension of initial presentation. Once the diagnosis is confirmed, an Belly/Pelvis CT is required for completion of staging, together with a torso PET. In this scenario, the examination is performed in 3 series: a depression dose Neck/Chest series, a diagnostic Abdomen/Pelvis series, and a small depression dose series of the extremities to lucifer the PET bed positions. As described earlier, these iii series are merged into a single data set and used for attenuation correction, fusion/anatomic co-localization with the PET, and diagnostic interpretation (Fig. 10).
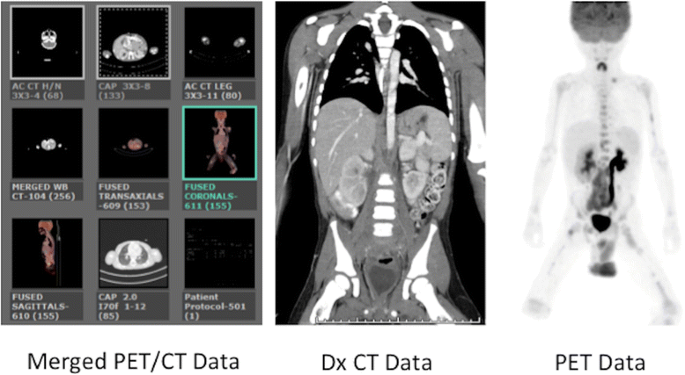
Multi-series PET/CT. Screen capture from the conquering workstation shows the components of the multi-series PET/CT test, in this instance including low dose AC CT coverage of the Head/Cervix, Dx CT coverage of the Chest, Abdomen, and Pelvis, and completion depression dose Air conditioning CT coverage of the upper legs to match the PET conquering. The merged CT data used to attenuation correct the PET data is displayed as a separate series at the workstation. Images from both the PET/CT and the diagnostic CT examinations are sent to their corresponding PACS exam folders and reported separately
PET/CT acquisition techniques vary considerably betwixt institutions [5]. One result of implementing our multi-series acquisition technique has been a reduction in radiation dose at our establishment. In phantom experiments, Fahey et al. showed that dose reductions of as much equally 44% tin be achieved using an integrated multi-series PET/CT acquisition technique, as compared to 2 separately acquired examinations [v]. In addition to reducing unnecessary radiation dose, having diagnostic quality CT data for paradigm co-registration and fusion can also improve the diagnostic accuracy of the PET examination and provide anatomic correlation for lesions that would have been difficult to discern on a depression dose attenuation correction CT.
In all cases, the CT used for attenuation correction, whether low dose or of diagnostic quality, should exist acquired with the same patient positioning and the same quiet animate used for the PET acquisition. This is peculiarly important for lesions in the chest and upper abdomen, where large differences in patterns of animate (e.one thousand. end-inspiration vs quiet breathing) can lead to mis-registration artifacts [22]. If an terminate-inspiratory diagnostic quality breast CT is required as part of diagnostic CT body exam, we volition oft obtain this separately at the end of the PET/CT test. Sedated patients will have similar quiet breathing patterns for both the CT and PET acquisitions and co-registered images can ordinarily be accurately generated without difficulty. We do non routinely intubate patients for the purposes of breath-holding during PET/CT.
PET acquisition
PET imaging is performed using standard techniques in accordance with the Northward American and EANM consensus guidelines and the 2016 update of the Due north American guidelines [28, 29], using administered activities of three.7-v.2 MBq/kg (0.10-0.xiv mCi/kg) for 18F-FDG, resulting in effective doses ranging from v.2-7.four mSv per test. Lower administered activities have also been used in an effort to generate sub-mSv PET examinations [xxx], however these usually crave longer acquisition times and have not been rigorously validated to ensure sensitivities and specificities that are comparable with the existing techniques.
Patients are placed in a warm injection room (~24 °C/75 ° F) for at to the lowest degree xxx min prior to FDG injection to reduce FDG uptake in brown adipose tissue. Others have reported using β-blockers, such as propranolol, depression dose benzodiazepine (diazepam), or short-acting opiates such every bit intravenous fentanyl in an effort to reduce brown fat uptake [22]. In our experience, proper patient preparation, with instructions to avoid common cold exposure and dress warmly (even during warm summer months, ambient temperatures in air conditioned cars and hospitals may be quite common cold for a lightly dressed child), followed by warming of the patient prior to, and during the uptake period, can essentially eliminate brown fat uptake in most patients. We exercise non routinely administer benzodiazepines, opiates, or other pharmaceuticals during the PET/CT examination, and in many institutions use of these agents is considered procedural sedation and requires prior consultation with anesthesia and/or sedation services. During the uptake menstruation patients are also instructed to minimize repetitive muscle activity in an effort to reduce background muscle uptake, although in practice the patterns of muscle uptake in children related to, for case, inconsolable crying, use of a pacifier, or utilize of electronic devices such as cell phones, can usually exist readily recognized and interpreted.
Following FDG injection, an uptake flow of 60 min is standard for virtually body imaging and oncologic applications. For brain tumor imaging, a shorter uptake menstruation can be utilized (30 min) simply should be standardized for all such studies. The PET/CT acquisition is ideally started as soon as possible after the 60 min tracer uptake menstruation. Wide variability in uptake times can affect the reproducibility and comparability of SUV values betwixt studies, although in practice it is challenging – specially for sedated patients – to adhere rigorously to the lx min uptake period guideline, and it is mostly accepted that absolute SUV values are prone to x-15% variability, related both to unavoidable differences in uptake time and variability in tissue biodistribution between examinations [31].
The PET/CT acquisition and reconstruction parameters will depend on the equipment bachelor. On our Siemens mCT xl PET/CT with a xx-cm axial field of view, PET images are acquired in 3D way using an conquering time of three min per bed position (Table iii), with the acquisition proceeding from head to toe. Reversing the direction of the PET acquisition, moving from feet to caput, may be indicated, particularly for bladder and pelvic neoplasms where excreted tracer in the bladder tin obscure tumor uptake, although every bit noted earlier, placement of a bladder catheter could be considered in such circumstances. Arms may exist positioned to a higher place the caput or be placed at the patient'southward side, depending on the indication for the exam. Head and cervix tumors are all-time imaged with the arms down to minimize CT beam-hardening artifacts in the neck. Similarly, tumors in the thorax and upper abdomen may benefit from having the arms up, although immature children often observe it difficult to hold their arms above the head for the ~20 min PET acquisition without some additional support (handles or the head holders supplied by near manufacturers). In practice, having the arms at the patient's side, slightly elevated off the bed to reduce the beam hardening that tin occur when upper artillery and the vertebral column are in alignment, is adequate for nigh pediatric indications, and preferred for sedated patients in whom having the arms higher up the caput may compromise the airway and IV access.
Iterative reconstruction of the PET data has been standard for more than than 15 years. However, the reconstruction of the 3D PET data volition require either a 3D reconstruction algorithm or rebinning of the information into a 2d set prior to reconstruction. Many sites utilize ordered subset expectation maximization (OSEM) iterative reconstruction leading to a reduction in reconstruction time. Lastly, we routinely utilize iterative reconstruction with resolution recovery which has improved the imaging of small structures, especially in our younger patients.
PACS integration
Once the PET/CT examination is consummate, attenuation correction of the PET imaging data and co-registration with the previously determined CT dataset is performed at the acquisition workstation. For routine examinations done without a diagnostic CT, the PET and attenuation correction CT exams are automatically candy and sent to the Picture Archiving and Communication System (PACS). When a diagnostic CT is existence integrated into the PET/CT exam, the respective depression dose and diagnostic CT serial are first merged, followed past attenuation correction of, and co-registration to, the PET data.
When sending the PET/CT information to the PACS system, at a minimum the post-obit series should be sent with every exam: 1) the attenuation-corrected PET exam, two) the not-attenuation corrected PET data, and 3) the co-registered CT images used for attenuation correction. Inclusion of the uncorrected PET information is important to allow credible focal areas of FDG uptake that are related to attenuation correction artifacts, rather than disease, to exist evaluated. Boosted series that nosotros routinely include with all PET/CT examinations include a maximum-intensity-projection (MIP) paradigm of the PET examination, axial, sagittal, and coronal fused PET/CT images, axial, sagittal, and coronal CT images reconstructed using a soft-tissue reconstruction kernel, and axial images of the thorax processed using a lung technique.
When a Dx CT has been integrated into the PET/CT examination, special considerations are needed to allow the diagnostic CT images to reside simultaneously in the PACS organization within both the PET/CT folder and the Dx CT binder. Near PACS systems prevent the same imaging data from being sent to two different destinations inside a given patient's test folder by assigning each acquisition serial a unique ID or UID. This is to forbid the inadvertent placement of duplicate exams in the patient'southward image archive, which can consequence in defoliation and potentially pb to erroneous image interpretation. Depending on both the PET/CT platform and the PACS vendor, a protocol must be developed to let the diagnostic CT data to co-exist within the PET/CT exam folder, either separately or as role of a merged CT dataset, and within the diagnostic CT folder.
For example, using the scenario described previously, in which a patient undergoes a torso PET with a Dx CT of the Abdomen/Pelvis, the following workflow is used:
- one)
Both the PET/CT Body and the Abdomen/Pelvis Dx CT examination orders are activated, which assigns PACS acquisition numbers to each of the orders.
- two)
Once the PET/CT is complete, the PET examination is processed using the merged CT data, equally detailed above. When the processing of the PET/CT exam is complete and the images have been sent to PACS, the Dx CT portion of the PET/CT examination is transferred into the Dx CT folder on the conquering workstation (this folder will just be available if the both the diagnostic CT and PET/CT orders take been activated prior to the test) then sent to PACS. Other strategies are possible, but we have plant this to exist an efficient workflow that minimizes procedural errors during the image transfer process.
It is besides the practise in many nuclear medicine departments to transport PET/CT images to a defended nuclear medicine viewing workstation to afford a more comprehensive review of the PET/CT data. At our institution, images are reviewed using the Hermes GOLD™ (Hermes Medical Solutions) viewing platform, although other vendors such as MIM Software, Inc. provide similar functionality. Well-nigh PACS vendors also include some blazon of PET/CT viewing capability inside the PACS surroundings (due east.g. Synapse three-D, GE Centricity Universal Viewer, etc.), which may exist adequate, depending on the clinical environment.
- 3)
In one case the exam has been completely processed and delivered to the appropriate PACS folders, the technologist performing the examination should confirm receipt of the right images in PACS and assure that both the diagnostic CT and PET/CT folders have the appropriate images needed for interpretation. Split up dictations are then issued for each of the corresponding examinations.
PET/CT interpretation
Estimation
Each department will have its ain preferred workflow for interpreting PET/CT examinations, although in practice most radiologists and nuclear medicine physicians review these circuitous examinations similarly.
All PET images should be reviewed in the transverse/axial, coronal and sagittal planes, reviewing both the fused and un-fused data. This is all-time accomplished using either a dedicated nuclear medicine processing and viewing workstation or a PACS-integrated nuclear medicine viewing functionality. Regardless of the viewing environs chosen, information technology is essential that PET epitome thresholds be scalable and adjustable. The fusion workstation should accept the capability of displaying fused images with dissimilar percentages of PET and CT blending, and should have the capability of measuring SUV, including use of volumetric ROI.
We generally review the PET component of the exam outset, using the greyscale images on an appropriately calibrated monitor. Below the PET images, the fused images are displayed, which allows for convenient anatomic co-localization of any PET abnormalities. The fused images are then separately reviewed in all three planes, followed by a dedicated review of the CT images in all three planes (whether low dose attenuation correction CT, diagnostic CT or merged hybrid CT images).
The CT images are as well reviewed at a diagnostic PACS workstation in all three planes, using soft tissue, os and lung windows, to evaluate for incidental/unexpected findings. If a Dx CT scan was requested and performed as part of the PET/CT examination, then the Dx CT should exist reported separately to remain compliant with regulatory and reimbursement requirements. The physicians interpreting the PET/CT or the Dx CT must satisfy institutional credentialing requirements, and in our exercise the radiologist interpreting the diagnostic CT is usually separate from the physician interpreting the PET/CT. Although is it not standard exercise in well-nigh institutions, we result a separate report for the attenuation correction CT portion of the test, making reference to any pertinent findings on the accompanying PET examination, and ensuring a thorough and comprehensive review of all the available imaging data.
Report generation
In full general, three reports are provided for each examination: the PET report, the attenuation correction CT report and, when applicable, a diagnostic CT written report.
PET study
In accordance with ACR and SNMMI practice guidelines, the PET report should contain a clarification of the radiopharmaceutical used, the administered activity and road of administration, serum glucose level, patient weight and fourth dimension of injection. Since this will be the primary study reviewed past the referring clinician, whatever boosted data, such as need for sedation or contrast reactions, should exist noted.
Description of any areas of abnormal FDG uptake should be noted, with relation made to any correlative findings on the CT images and with provision of any quantitative or semi-quantitative measures of FDG aggregating (SUV). Any image artifacts or technical problems that could lead to paradigm misinterpretation should also be noted.
Attenuation correction CT
Although non standard practice or required past the guidelines, it is our practise to review the attenuation correction CT images with the same rigor used for reviewing a diagnostic CT. A standardized reporting template is used, with brief descriptions of whatever CT findings that correlate with PET abnormalities, and annotation fabricated of whatsoever pertinent incidental or unexpected findings (e.g. spondylolysis, vertebral pinch, malpositioned support lines or catheters, etc).
Diagnostic CT
This is reported by the roofing body radiologist using standard dictation templates, in a similar manner to a Dx CT being obtained without an accompanying PET examination. In most cases the radiologist reviews the Dx CT together with the individual interpreting the PET exam, which insures a comprehensive review and allows both the PET and Dx CT reports to convey compatible information.
Artifacts
A give-and-take of the numerous artifacts that i may come across during PET/CT examinations is beyond the telescopic of this review and has been described previously [22]. Where indicated strategies for minimizing specific artifacts or physiologic variants (i.eastward. muscle and brown fat uptake) have been noted elsewhere throughout this review.
Conclusions
PET/CT plays an of import role in the direction of many pediatric malignancies. This review provides an overview of our approach to determining when and how a PET/CT test is performed, with attention to optimizing the CT component of the PET/CT test. A multi-disciplinary approach is needed to ensure that the indications for PET/CT are appropriate, that the right exam is performed, and the advisable imaging techniques are used. Equally hybrid imaging technologies such as PET/CT have evolved, new strategies for image acquisition and interpretation accept emerged, leading to many exciting opportunities for improving the overall quality of the PET/CT experience.
References
-
Freebody J, Wegner EA, Rossleigh MA. 2-deoxy-2-((18)F)fluoro-D-glucose positron emission tomography/computed tomography imaging in paediatric oncology. World J Radiol. 2014;6(10):741–55.
-
Harrison DJ, Parisi MT, Shulkin BL. The part of 18F-FDG-PET/CT in pediatric sarcoma. Semin Nucl Med. 2017;47(3):229–41.
-
Kluge R, Kurch 50, Georgi T, Metzger M. Current part of FDG-PET in pediatric Hodgkin's lymphoma. Semin Nucl Med. 2017;47(three):242–57.
-
Uslu L, Donig J, Link One thousand, Rosenberg J, Quon A, Daldrup-Link HE. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. 2015;56(2):274–86.
-
Fahey FH, Goodkind A, MacDougall RD, Oberg L, Ziniel SI, Cappock R, Callahan MJ, Kwatra Northward, Treves ST, Voss SD. Operational and Dosimetric aspects of pediatric PET/CT. J Nucl Med. 2017;58(9):1360–366.
-
Parisi MT, Bermo MS, Alessio AM, Sharp SE, Gelfand MJ, Shulkin BL. Optimization of pediatric PET/CT. Semin Nucl Med. 2017;47(3):258–74.
-
Jacques L, Jensen TS, Rollins J, Caplan S, Roche JC: Decision memo for positron emission tomography (FDG) for solid tumors (CAG-00181R4). Edited by Services CfMaM; 2013.
-
Flerlage JE, Kelly KM, Beishuizen A, Cho S, De Alarcon PA, Dieckmann U, Drachtman RA, Hoppe BS, Howard SC, Kaste SC, et al. Staging evaluation and response criteria harmonization (SEARCH) for childhood, boyish and young adult Hodgkin lymphoma (CAYAHL): methodology statement. Pediatr Blood Cancer. 2017;64(seven):e26421/ane-ten.
-
Hawkins DS, Conrad EU tertiary, Butrynski JE, Schuetze SM, Eary JF. [F-xviii]-fluorodeoxy-D-glucose-positron emission tomography response is associated with consequence for extremity osteosarcoma in children and young adults. Cancer. 2009;115(15):3519–25.
-
Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad Eu 3rd, Eary JF. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23(34):8828–34.
-
Raciborska A, Bilska Thou, Drabko One thousand, Michalak E, Chaber R, Pogorzala M, Polczynska G, Sobol Thou, Wieczorek Grand, Muszynska-Roslan K, et al. Response to chemotherapy estimates by FDG PET is an of import prognostic factor in patients with Ewing sarcoma. Clin Transl Oncol. 2016;18(ii):189–95.
-
Tsai LL, Drubach L, Fahey F, Irons M, Voss South, Ullrich NJ. [18F]-Fluorodeoxyglucose positron emission tomography in children with neurofibromatosis type 1 and plexiform neurofibromas: correlation with malignant transformation. J Neuro-Oncol. 2012;108(3):469–75.
-
Kiratli PO, Tuncel M, Bar-Sever Z. Nuclear medicine in pediatric and adolescent tumors. Semin Nucl Med. 2016;46(four):308–23.
-
Franzius C. FDG-PET/CT in pediatric solid tumors. Q J Nucl Med Mol Imaging. 2010;54(4):401–x.
-
Dumba M, Jawad Northward, McHugh K. Neuroblastoma and nephroblastoma: a radiological review. Cancer Imaging. 2015;15(5):1–14.
-
Sharp SE, Trout AT, Weiss BD, Gelfand MJ. MIBG in Neuroblastoma diagnostic imaging and therapy. Radiographics. 2016;36(1):258–78.
-
Sharp SE, Shulkin BL, Gelfand MJ, Salisbury S, Furman WL. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med. 2009;50(8):1237–43.
-
Moinul Hossain AK, Shulkin BL, Gelfand MJ, Bashir H, Daw NC, Sharp SE, Nadel Hour, Dome JS. FDG positron emission tomography/computed tomography studies of Wilms' tumor. Eur J Nucl Med Mol Imaging. 2010;37(7):1300–8.
-
Weiser DA, Kaste SC, Siegel MJ, Adamson PC. Imaging in childhood cancer: a Society for Pediatric Radiology and Children's oncology grouping joint task force report. Pediatr Blood Cancer. 2013;lx(8):1253–60.
-
Wong TZ, Paulson EK, Nelson RC, Patz EF Jr, Coleman RE. Applied approach to diagnostic CT combined with PET. AJR Am J Roentgenol. 2007;188(3):622–ix.
-
Osman MM, Cohade C, Fishman EK, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352–5.
-
Grant FD. Normal variations and benign findings in pediatric 18F-FDG-PET/CT. PET Clin. 2014;ix(2):195–208.
-
Schafer JF, Gatidis S, Schmidt H, Guckel B, Bezrukov I, Pfannenberg CA, Reimold M, Ebinger Chiliad, Fuchs J, Claussen CD, et al. Simultaneous whole-body PET/MR imaging in comparing to PET/CT in pediatric oncology: initial results. Radiology. 2014;273(1):220–31.
-
Gatidis S, Schmidt H, Gucke B, Bezrukov I, Seitz Chiliad, Ebinger K, Reimold M, Pfannenberg CA, Nikolaou K, Schwenzer NF, et al. Comprehensive oncologic imaging in infants and preschool children with substantially reduced radiations exposure using combined simultaneous (ane)(viii)F-Fluorodeoxyglucose positron emission tomography/magnetic resonance imaging: a straight comparison to (1)(8)F-Fluorodeoxyglucose positron emission tomography/computed tomography. Investig Radiol. 2016;51(1):vii–14.
-
Gatidis Southward, Bender B, Reimold M, Schafer JF. PET/MRI in children. Eur J Radiol. 2017;94:A64–A70.
-
Aschoff P, Plathow C, Beyer T, Lichy MP, Erb G, Oksuz MO, Claussen CD, Pfannenberg C. Multiphase contrast-enhanced CT with highly concentrated contrast agent tin can be used for PET attenuation correction in integrated PET/CT imaging. Eur J Nucl Med Mol Imaging. 2012;39(2):316–25.
-
Berthelsen AK, Holm Southward, Loft A, Klausen TL, Andersen F, Hojgaard 50. PET/CT with intravenous dissimilarity tin can exist used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging. 2005;32(ten):1167–75.
-
Treves ST, Gelfand MJ, Fahey FH, Parisi MT. 2016 update of the north American consensus guidelines for pediatric administered radiopharmaceutical activities. J Nucl Med. 2016;57(12):15N–8N.
-
Lassmann M, Treves ST, Group ESPDHW. Paediatric radiopharmaceutical assistants: harmonization of the 2007 EANM paediatric dosage carte du jour (version 1.5.2008) and the 2010 north American consensus guidelines. Eur J Nucl Med Mol Imaging. 2014;41(5):1036–41.
-
Hope T, Phelps A, Behr South, Seo Y, Corbin Chiliad, MacKenzie J. Sub-mSv whole body PET/MRI in pediatric patients. Pediatr Radiol. 2017;47(Suppl 1):163.
-
Social club MA. Repeatability of SUV in oncologic 18F-FDG PET. J Nucl Med. 2017;58(4):523–32.
-
Mossé YP, Voss SD, Lim MS, Rolland D, Minard CG, Play a trick on E, Adamson P, Wilner Grand, Blaney SM, Weigel BJ. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Jail cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol. 2017; 35(28):3215–21.
Acknowledgements
Richard Powers, Ph.D., Siemens Healthcare, for his contribution to the development of the multi-series PET/CT software described in this review.
Funding
No external sources of funding were used.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Affiliations
Contributions
All authors contributed to preparation, editing, and final approval of the submitted manuscript.
Respective author
Ideals declarations
Ethics approval and consent to participate
The requirements for a formal ethics board review and for patient consent to participate were waived by the Boston Children'due south Hospital IRB based on the retrospective nature of this article and the lack of any identifiable patient data.
Consent for publication
Non applicable. No individual person's data or identifiable data is presented.
Competing interests
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed nether the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/past/iv.0/), which permits unrestricted apply, distribution, and reproduction in whatever medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Artistic Commons license, and signal if changes were made. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data fabricated bachelor in this article, unless otherwise stated.
Reprints and Permissions
Nearly this commodity
Cite this commodity
Colleran, G.C., Kwatra, Northward., Oberg, L. et al. How we read pediatric PET/CT: indications and strategies for epitome acquisition, estimation and reporting. Cancer Imaging 17, 28 (2017). https://doi.org/ten.1186/s40644-017-0130-8
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s40644-017-0130-8
Keywords
- PET/CT
- Diagnostic CT
- Pediatric oncology
- Hybrid imaging
- Dose reduction
- Attenuation correction
- Multidisciplinary interpretation
Source: https://cancerimagingjournal.biomedcentral.com/articles/10.1186/s40644-017-0130-8